Lithium-ion battery, how does it work?
Lithium-ion batteries are extremely popular and versatile. Found in cell phones, automobiles, power tools, and several other types of electronic devices,
Lithium-ion batteries are extremely popular and versatile. Found in cell phones, automobiles, power tools, and several other types of electronic devices, these rechargeable batteries are also making an impact on powering material handling and airport ground support equipment.
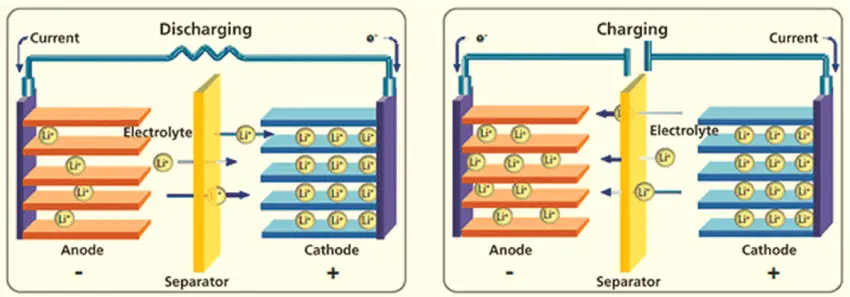
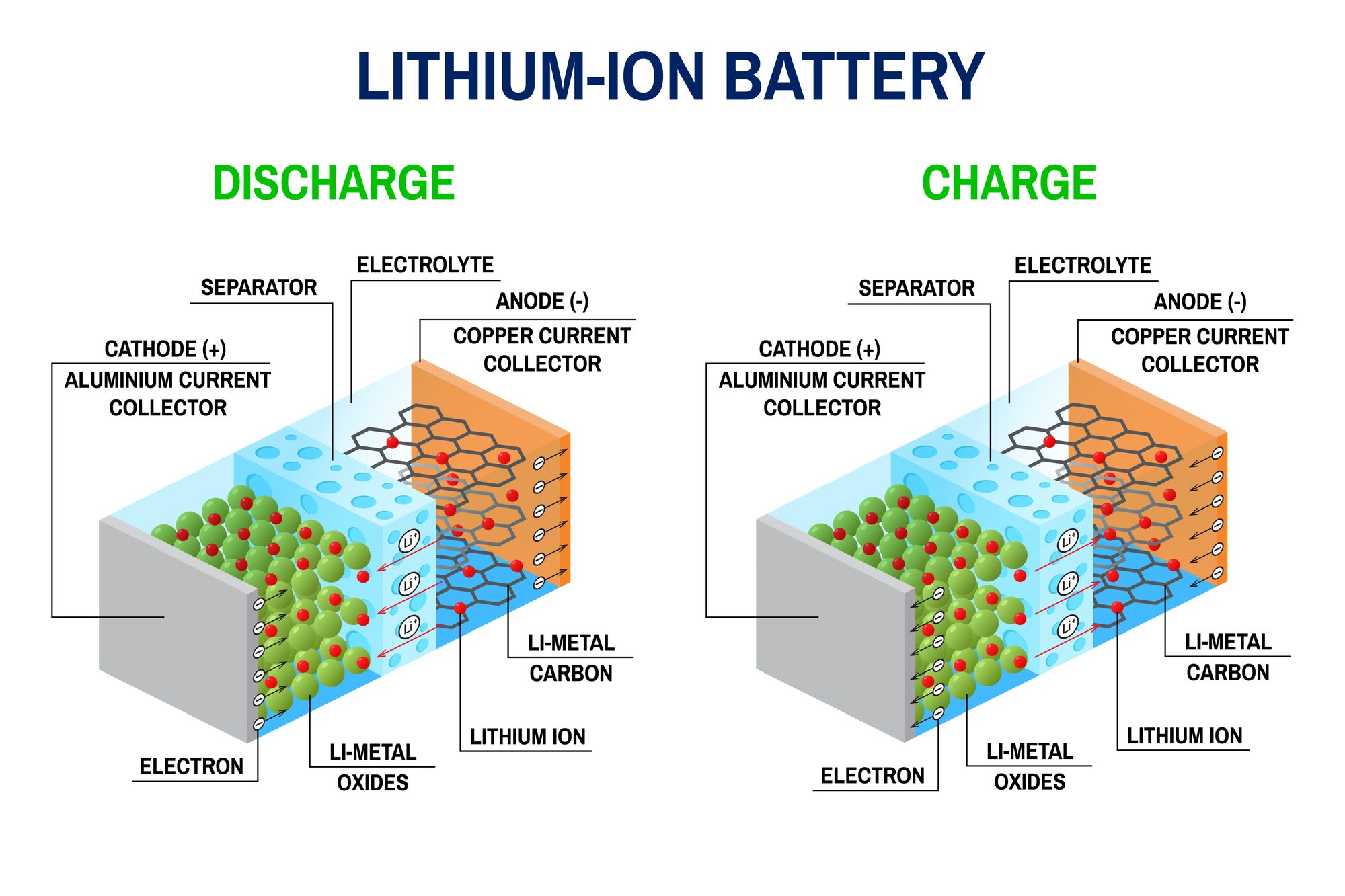
A battery has an anode, cathode, separator, electrolyte, and two current collectors (positive and negative). The anode and cathode store the lithium. The electrolyte carries positively charged lithium ions from the anode to the cathode and vice versa through the separator. The movement of the lithium ions creates free electrons in the anode that creates a charge at the positive current collector. The electrical current heathen flows from the current collector through a device being powered (cell phone, computer, etc.) to the negative current collector. The separator blocks the flow of electrons inside the battery.
Lithium-ion first came to the market in 1912 under G.N. Lewis, but it was not until the early 1970s that the first non-rechargeable lithium batteries became commercially available. Attempts to develop rechargeable lithium batteries followed in the 1980s but failed because of instabilities in the metallic lithium used as anode material. Because the metal-lithium battery uses lithium as anode; Li-ion uses graphite as anode and active materials in the cathode.
Lithium is the lightest of all metals, has more electrochemical potential, and provides the largest specific energy per weight. Rechargeable batteries with lithium metal on the anode could provide extraordinarily high energy densities; however, it was discovered in the mid-1980s that cycling produced unwanted dendrites on the anode. These growth particles penetrate the separator and cause an electrical short. The cell temperature would rise quickly and approach the melting point of lithium, causing thermal runaway, also known as “venting with flame.” A large number of rechargeable metallic lithium batteries sent to Japan were recalled in 1991 after a battery in a mobile phone released flaming gases and inflicted burns to a man’s face.
The technology behind lithium-ion batteries makes them a great choice because of their distinct advantages and environmentally-friendly benefits. But, how exactly do lithium-ion batteries work? And, what makes them so popular in so many applications? Here’s what you need to know about the components that make up a lithium-ion battery and how they work together to create high-functioning and long-lasting sources of power.
The components
Lithium-ion batteries are available in many different shapes and sizes. Inside, however, they typically look the same. In order to understand how the battery works, you need to know the role that each part plays.
a) The Cell
A lithium-ion battery is made up of several parts. The cell, serving as the workhorse of the battery, is the most critical component of the battery.
The cell comprises the following battery materials:
- Electrodes are the two battery ends. One is the anode, and the other is the cathode.
- The anode stores lithium made from carbon.
- The cathode also stores lithium and is made from a chemical compound; a metal oxide.
- The separator blocks the flow of negative and positive electrons inside the battery but allows ions to pass through.
- The electrolyte liquid sits between the two electrodes. It carries the positively charged lithium ions from the anode to the cathode and vice versa depending on whether the battery is charging or discharging.
b) The Battery Pack
The battery pack, which holds the lithium-ion cells, operates much like a computer. It contains the following:
- At least one temperature sensor to monitor the battery’s temperature.
- A voltage converter and regulator circuit that focuses on keeping the voltage and current at safe levels.
- A Euro connector, allows power and information to move in and out of the battery pack.
- The cell tap oversees the cells’ voltages in the battery pack.
- A battery monitoring system is a small computer that oversees the whole battery and ensures safety to the user.
Movement in the Cell
When you plug a lithium-ion battery into a device the positively-charged ions move from the anode to the cathode. As a result, the cathode becomes more positively charged than the anode. This, in turn, attracts negatively-charged electrons to the cathode.
A separator in the cell includes electrolytes that form a catalyst. This promotes ion movement between them. The movement of ions through the electrolyte solution is what causes the electrons to move through the device the battery is plugged into.
Lithium-ion batteries are rechargeable. When recharging, the lithium ions go through the same process, but in the opposite direction. This restores the battery for additional use.
The overall design of a lithium-ion battery provides many benefits for equipment users:
- Run times dramatically increase with their use compared to other battery types.
- Fast-charging capabilities provide less downtime for shift workers and greater productivity.
- They feature flat discharge curves and provide higher constant power. It means no more annoying sluggishness in equipment as the battery charge level decreases.
Battery Management System (BMS)
The management system plays an integral role in making sure the battery cell works at its highest levels. It also impacts how the battery functions, offering several protections and features.
For example:
- The BMS maintains cell temperatures in the ideal operating range to prevent overheating or freezing.
- The BMS monitors currents and voltage to keep both at safe levels. Dendrites begin to form in the cell if voltages drop too low which can short the cell, so a lithium-ion battery pack must have a system to monitor this.
- There is no “memory” built into the pack, so partial discharges do not hurt the battery. Lithium-ion batteries can charge and discharge during the times that are most convenient for equipment operators.
- Built-in controllers prevent overcharging, protect from forming that can cause significant damage to lithium-ion batteries.
- Cell balancing is monitored so that equalization charges are never needed. Because lithium-ion batteries do not need equalization charges, they do not release dangerous gasses.
- The battery management system also allows managers to track the battery health of their fleet through onboard computers that send vital data through cloud-based services.
Advantages of Lithium-ion
- High specific energy and high load capabilities with Power Cells
- Long cycle and extended shelf-life; maintenance-free
- High capacity, low internal resistance, good coulombic efficiency
- Simple charge algorithm and reasonably short charge times
- Low self-discharge (less than half that of NiCd and NiMH)
Limitations of Lithium-ion
- Requires protection circuit to prevent thermal runaway if stressed
- Degrades at high temperature and when stored at high voltage
- No rapid charge possible at freezing temperatures (<0°C, <32°F)
- Transportation regulations are required when shipping in larger quantities
References:
i) Justin. F (2019) How Does A Lithium-ion Battery Work?




